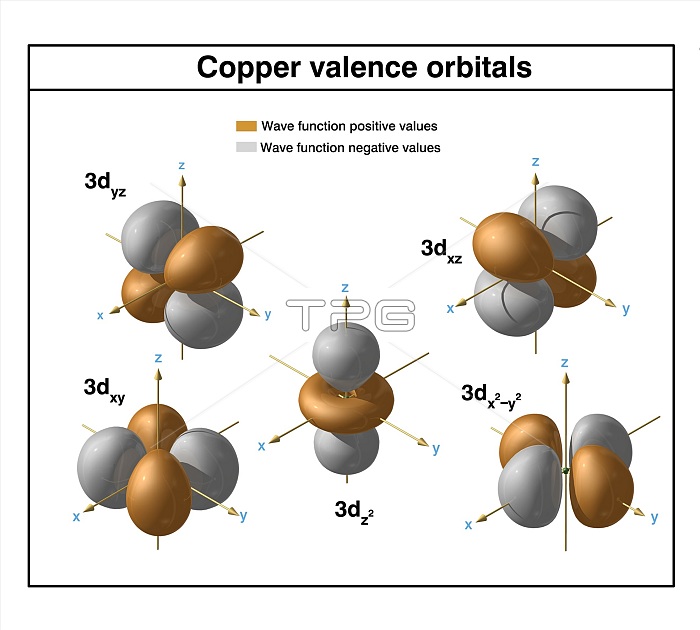
Copper (Cu). Diagram of the valence orbitals of an atom of copper-64 (atomic number: 29), an isotope of this element. Copper is a transition metal in group 11, period 4, and the d-block of the periodic table. It has a melting point of 1084 degrees Celsius. The trends across the transition metals are due to electrons filling an inner d-subshell, shielding the outer (valence) electrons from the increasing nuclear charge.
| px | px | dpi | = | cm | x | cm | = | MB |
Details
Creative#:
TOP24702958
Source:
達志影像
Authorization Type:
RM
Release Information:
須由TPG 完整授權
Model Release:
N/A
Property Release:
N/A
Right to Privacy:
No
Same folder images:
3D3D104S4S1ATOMICATOMICORBITALSCHEMICALDBLOCKD-BLOCKELECTRONSELEMENTALELEMENTSGROUP11METALMETALLICMETALSORBITALORBITALTYPESORBITALSPERIOD4QUANTUMCHEMISTRYSHELLSHELLSTRUCTURESHELLSSOLIDSOLIDSSTRUCTURALSTRUCTURESUB-ATOMICSUBATOMICSUBSHELLSUBSHELLSTRANSITIONMETALTRANSITIONMETALSVALENCEORBITALWAVEFUNCTIONWHITEBACKGROUNDISOTOPEELEMENTATOMELECTRONCONFIGURATIONELECTRONSHELLCHEMISTRYPHYSICALCHEMISTRYILLUSTRATIONDIAGRAM
113D3D1044S4S1ATOMATOMICATOMICBACKGROUNDBLOCKCHEMICALCHEMISTRYCHEMISTRYCHEMISTRYCONFIGURATIONDD-BLOCKDIAGRAMELECTRONELECTRONELECTRONSELEMENTELEMENTALELEMENTSFUNCTIONGROUPILLUSTRATIONISOTOPEMETALMETALMETALLICMETALSMETALSORBITALORBITALORBITALORBITALSORBITALSPERIODPHYSICALQUANTUMSHELLSHELLSHELLSHELLSSOLIDSOLIDSSTRUCTURALSTRUCTURESTRUCTURESUB-ATOMICSUBATOMICSUBSHELLSUBSHELLSTRANSITIONTRANSITIONTYPESVALENCEWAVEWHITE
 Loading
Loading